Introduction: Choosing Greener Metals
So you're interested in choosing greener metals for your geekadelic project. Or even better, you're making Eco-geek stuff! Either way, how do you choose the most sustainable metals?
Step 1: Raid the Scrap Bin!
When you go to your local metal store, raid the scrap bin!
If you're saving something from landfill, you can argue it's "ecologically free". Don't get too self-righteous about this, but it's a pretty good rule of thumb. And scrap is a LOT cheaper than virgin metal cut to size!

Almost any metal store will have a scrap bin, and there're lots of specialty scrap dealers around. (Their stores are way more fun to walk through, too!) Making your stuff out of scrap might save you time, too—why build parts from scratch that you could just find?

(Above) Scrap-metal bird from the Forevertron
Step 2: Buy Recycled Metal If You Can.
The heat and electricity used to refine ores are often (though not always) the biggest environmental impacts of making metals. Aluminum is sometimes called "solidified electricity" because it takes so much energy to make it from its ore (bauxite).
Recycled metal avoids this problem, and it can make a big difference—recycling aluminum only uses about 1/20th the energy as making virgin aluminum. Even for steel, recycling uses about 1/4th the energy as making virgin steel.
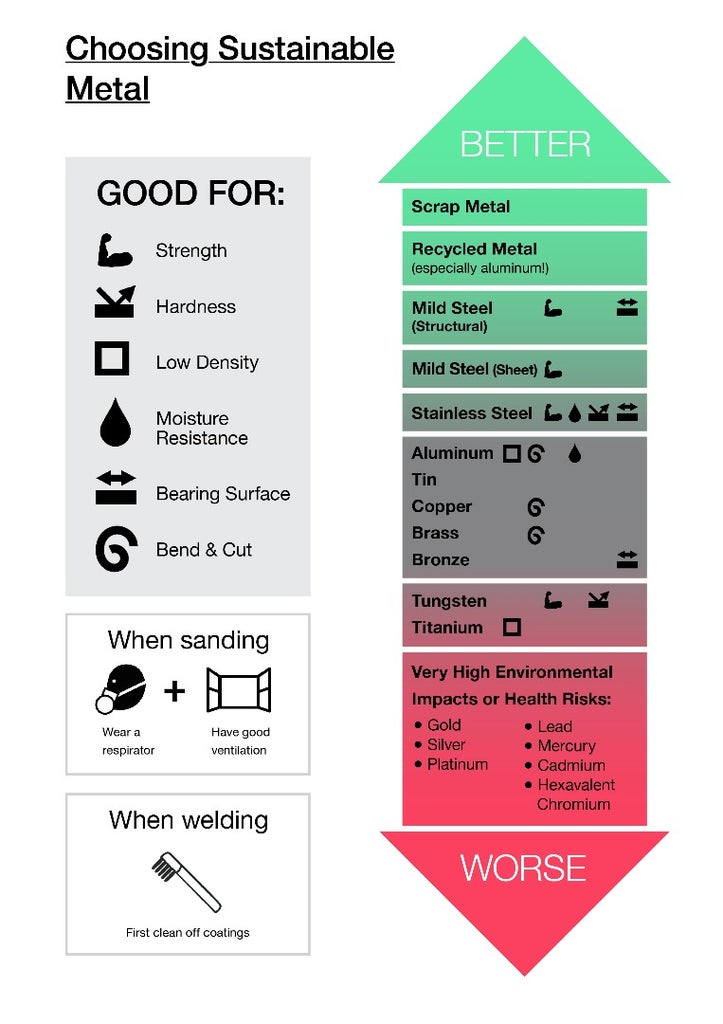
So who sells recycled? Almost all structural steel (I-beams, etc.) is 95%+ recycled. But somehow it's really hard to find sheet steel with more than 25% recycled content. If you know someone who works at a steel mill, bug them about this. It's dumb. For aluminum, it'll depend by alloy. Ask your local shops.

Step 3: What's a Greener Metal?
By far most of the metal you're likely to build with is steel and aluminum. The good news is that most of the time, cheap metals are green metals, and expensive metals aren't. Iron and mild steel are not only the cheapest metals you can get, but also the least energy- and resource-intensive metals. (A great paper from MIT shows how people only need to dig up 2-5 lbs of ore for every 1 lb of iron, using only about 20-30 MJ/kg of embodied energy.) It's also by far the most-recycled metal. And it isn't toxic, by itself.

Aluminum also isn't toxic (the Alzheimer's thing was a myth from bad science), and while virgin aluminum does take a lot of energy (200-300 MJ/kg), it's still less energy than many other metals (like titanium), and it's easy to recycle.

Compare these to the precious metals: Gold takes 100,000 to 10,000,000 pounds of ore for every 1 pound of gold you buy, using about 100,000 MJ/kg of embodied energy. And while gold itself isn't toxic to you once it's refined, lots of arsenic is used to separate the gold from the ore, which leaves lots of toxic crap for mines, miners, and their local communities to handle. Silver isn't as bad, but it's still about 10,000 times as resource-intensive and 10x more energy-intensive than aluminum. Platinum is even worse than gold.
Copper is surprisingly bad environmentally—partly because it's increasingly scarce (every pound of copper these days requires 100 – 1,000 pounds of ore), and partly because it's surprisingly toxic in rivers and lakes. It's regularly used in pesticides and wood preservatives because of its toxicity. (Though it takes a fairly significant dose to be toxic to you, and trace amounts are necessary for your health.) Copper's not nearly as bad as gold, but if you can use steel instead, do it. Or if you're plumbing, some non-PVC plastics like PEX or polypropylene are much better.

Brass and bronze are copper alloys. The other metal in brass is zinc, which
unfortunately has even higher eco-toxicity than copper (again, it's not toxic to people, just to other animals & plants). But for bronze, the other metal is tin, which has lower impacts, so it's a slightly greener metal than pure copper.

Stainless steel has higher eco-impacts than mild steel, but less than aluminum or most other metals. Although a couple of its ingredients (chromium and nickel) can be toxic to people, they're so bound up in the alloy that it's really safe, even for food. It's so safe, most high-strength medical implants are made with it. Also, it's so much harder and so much more corrosion-resistant than mild steel that it ends up being a better choice for many projects, durability-wise.

Here's a little chooser chart of preferred metals. You can also open a PDF version of the chart below.
Attachments
Step 4: Make Sure It's Up to the Job.
If your project breaks, that's not very green, no matter how eco-friendly the materials are. So make sure you choose a metal with the right physical properties for you!
Are you making something structural, like a bed or table? Then you care about strength (not breaking under force). Are you making a nice surface finish, like a counter? Then you care about hardness (resistance to dings and scratches). Are you making a vehicle? Then you care about density (to reduce weight). Will your project be outside? Then you care about weather-resistance (rust or corrosion). There might be other physical qualities important for your particular project, too.

Mild steel is strong and stiff, but not hard or corrosion-resistant—great for structural stuff, but the finish can get dinged, and it rusts easily, so it's not great for outdoors or food and drink unless it's painted or otherwise coated. (Unless you live someplace really dry, or don't mind rust.) Steel is also easy to weld, though it's not as easy to cut or bend as aluminum.

Stainless steel is every bit as strong and stiff as mild steel (often more so), plus it's also hard and super-corrosion-resistant. Since environmentally speaking it's usually better to avoid coatings like mild steel needs outdoors, stainless is a great choice for projects that'll be exposed to weather. Very low-maintenance. A simple brushed stainless finish looks great, too. And you can eat & drink out of it! The disadvantage of stainless is that because it's so wonderfully strong, stiff, and hard, it's difficult to bend and cut.

Aluminum isn't as strong or stiff as steel, but it's so much lighter (1/3 the density!) that you can make stronger structures for less weight, just by using thicker walls. And it's much easier to bend and cut than steel. It's a little harder to weld, but not that bad. So it's great for bicycles and other vehicles. The main downside for vehicles is that aluminum doesn't have a fatigue limit like steel does, but that only matters if your project is going to last a very long time or be hit with tons of vibration / impact / flexing. Aluminum doesn't rust in a structurally-bad way, but it does oxidize, so you get smudgy-looking fingerprints. You might want to anodize it to keep it looking nice. (Also, you might want to anodize aluminum you're eating or drinking out of, but the science is inconclusive about whether it really matters.)

(Above) Massive aluminum block at the Hanford LIGO observatory
Bronze is worth mentioning too, because even though it's not as green a metal as aluminum or steel, it can be very useful for bearing surfaces, handling more weight than plastic bushings could. Sliding aluminum on aluminum has horrible friction, and even steel on aluminum or steel on steel aren't great (useful table here), but bronze is good for both steel and aluminum to slide on. Brass is ok, too, but bronze is better.
Step 5: Choose Good Coatings, or Keep the Metal Naked!
Choosing a good metal is the most important part of the process, but sometimes the coating on the metal can have big environmental impacts, too. Like galvanized steel studs in buildings.
Galvanizing is surprisingly nasty. Thin-walled sheet steel with thick galvanization (like some steel studs) can actually have their environmental impacts from galvanization be as big as the mining, refining, and shaping the steel put together. It's because galvanization is a layer of zinc, which as I mentioned before is toxic to aquatic beasties. And the process of coating the steel with the zinc also involves acids and generates toxic byproducts that companies have to deal with carefully. (Some companies galvanize with thermal spraying instead, but that just trades high chemical impacts for high energy impacts.) So avoid galvanization when you can.

Some steel is rustproofed by tin plating rather than zinc. ("tin cans" are actually steel cans plated in tin.) This is much more eco-friendly than galvanizing, so if you can find it, use it. But it's hard to find outside of tin cans, and if it gets scratched, it rusts faster in that spot.
Paint is low-impact in terms of energy and resources, but it often contains toxins, some of which affect you directly by offgassing volatile organic compounds ("VOCs"). The way paints go from liquid to solid is by having some solvent that evaporates. But some solvents are much healthier than others—look for "no VOC" or "low VOC" paints. "Natural" paints like milk paint (yes, made out of milk!) are even better, though often not weatherproof. And don't just dump unused paint down the drain unless you know it's non-toxic! A good starter list of low-toxicity paints is here.

Do the right thing with paint waste: latex paint is recyclable, while oil-based paint is household hazardous waste. Find out how your city handles these things—in some places, latex paint can actually be recycled curbside! (Just let it sit out to dry completely first.) In other cities you can drop it off at a paint recycling center. And hazardous waste usually has to be taken somewhere (don't just dump it down the drain!)
Powder-coating isn't a liquid—it's a plastic powder that gets sprayed on (just sticking by static cling) and then gets baked to melt it into a single solid layer. The baking of course uses energy, but overall it's a smaller eco-impact than painting (particularly for you). Even better, powder coating is usually a much harder (more scratch-resistant) coating than paint. That's why it's popular on bikes.

For aluminum, you can also have it clear-anodized instead. There're lots of small vendors that'll do this for you. You could even leave it bare, but bare aluminum gets smudgy when handled, and can get your hands a little smudgy too. You might think that Apple laptops have naked aluminum shells, but they're all clear-anodized.
Smudgy bare aluminum vs. clear-anodized aluminum:
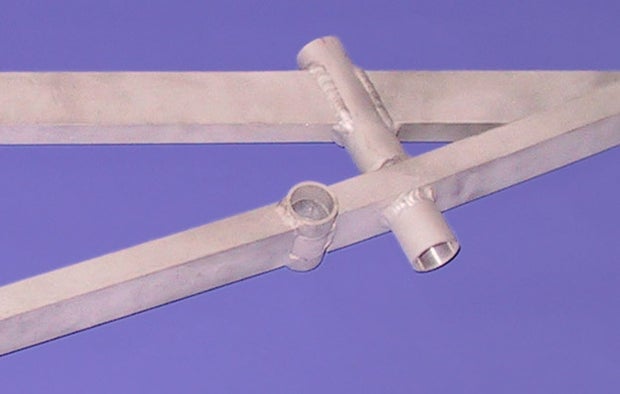
(Above) Smudgy aluminum
(Below) Clear-anodized aluminum

Anodization is a microns-thin layer of oxidation on the surface of the aluminum. Usually it's chemically benign, though some colored anodization uses nasty chemicals to get certain colors (like sulfuric acid, nickel acetate, even hexavalent chromium. Even though the final coating is non-toxic to you, you should still avoid these because the people doing the anodizing would have to deal with those chemicals. Clear-coat anodization is good, as are some colors.
Anodization also doesn't hurt the recycling value of the aluminum like paint and powder coating can (according to the three recyclers I talked to). It's such a thin coating (and even that coating is mostly aluminum itself) that anodized parts can be thrown right in with bare parts in recycling furnaces, with no extra hassle or emissions-scrubbing required.
Step 6: Working With Metal
The green way to work with metal is to be safe: don't poison yourself with dust and fumes. For some sanding you don't need to worry too much, because metal dust is pretty heavy and doesn't hang in the air that much, but the finer you're sanding, the finer the dust will be. Even if the metal itself isn't bad for you chemically, any particulates in your lungs are generally bad. Wearing a good dust mask will avoid this.
For welding, you always want to sand any paint or galvanization off the area first, out to a couple inches around where you'll be welding. Otherwise you'll vaporize the paint or zinc coating and breathe in toxic crap. You could instead have good ventilation and use a respirator, but you'll also get a much better weld if you're welding clean bare metal to metal.
For welding Aluminum, remember you should heat-treat it afterwards to get its strength back. Welding aluminum usually reduces its strength by messing up the crystal structure.

Other than sanding & welding, there's just one small surprising thing: to keep your metals lasting a long time, particularly in weather or water, don't put galvanically different metals together.
What're galvanically different metals, you ask? Good question. It's weird. Check it out on Wikipedia. In a nutshell, one metal can corrode over time because it's touching the wrong metal. Home plumbing where copper pipes meet mild steel pipes make the steel corrode many times faster than it would if it didn't touch the copper. But having steel touch tin is fine. Or if steel touches zinc, the zinc corrodes. (Which is why galvanizing works.) The Wikipedia article has a good table of what metals are ok with each other.














