Introduction: How to Make a Voltaic Pile - the World's First Battery
Background
The Voltaic Pile was the world's first electrical battery that could sustain electrical current to a circuit. Its creation was first published by Alessandro Volta in 1799, although it could have been made as early as several years prior.
The voltaic pile shares many similarities to the common lemon battery. While neither are very useful in today's world of mass-produced, high efficiency, and rechargeable batteries, the voltaic pile is a great demonstrative tool that teaches circuitry, voltage, parallel and series wiring, chemistry, reactions, and many more very useful concepts. This guide is not meant to be a mere step-by-step guide on how to make a voltaic pile (the actual construction device is fairly straightforward), but rather a guide by which to learn about the concepts at play, and how one might use these concepts to teach others, especially teachers teaching their pupils.

A Voltaic Pile, likely made by Volta's friend and scientific peer Luigi Galvani c. 1800-1820
About the Author
I am Christopher, I run Christopher's Factory, which encompasses channels on many platforms that create content based around science, technology, engineering, and mathematics, with an emphasis on making these concepts accessible to everyone. In 2023, learning new hobbies and skills has never been more within reach, and it is my goal to show everyone just how fulfilling it is to try new things.
Supplies
You will need...
- Sheets of copper and zinc
- These can be purchased in pre-cut strips for our exact purpose, which I highly recommend (Link)
- 7/32 x 1-1/2" Compression Springs
- If you enjoy making things, I recommend you buy a spring kit. This one contains the ones needed for this project, and many other common and useful types (Link)
- Table salt
- Water
- Access to a 3D printer and filament
- Wire
- Cardboard, tissue, toilet paper, or any other porous material that can hold liquid
Step 1: What Is a Battery?
For comedic relief, consider watching the Breaking Bad Battery Scene before reading further.

Photo: Sony Pictures Television
Electricity, at its most basic principle, is nothing more than the flow of electrons. By having two wires that differ in charge, we can cause work to be done at the point that they meet. A battery is a system that provides a continuous supply of electrons to be used by the circuit.
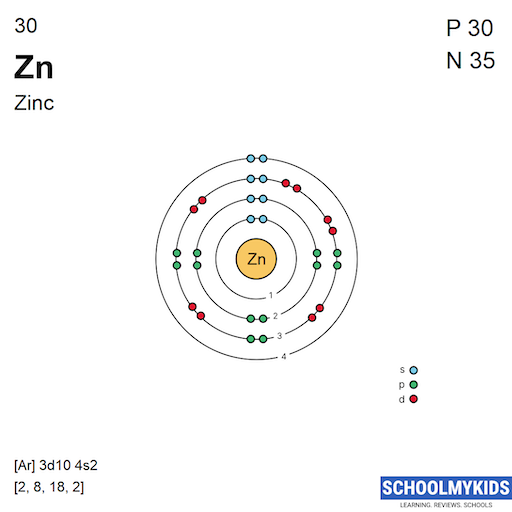
Zinc is an element that normally contains 30 electrons, two of which lie in the outermost energy level. Electrons in this outer shell are called valence electrons, and the number of valence electrons in this outer shell is a major determinant of the atom's behavior. This is why elements on the periodic table are grouped by number of valence electrons -- they tend to behave similarly.
Electrolytes are substances that have a natural charge (either positive or negative) when dissolved in water. When in the presence of an electrolyte, such as lemon juice or salt water, the zinc atoms readily shed their two valence electrons. This is a process known as oxidation, and is the same thing that happens when iron (or many alloys containing it) are left outside for long periods of time.
When iron is left outside, the metal reacts with the moisture in the air and gains what we call rust. The iron atoms change into iron oxide. The iron loses some of its valence electrons to the oxygen, which bonds with the iron and the hydrogen in the moisture to form hydrated iron oxide. Similarly, when zinc atoms are exposed to salt water, they shed their valence electrons, and are no longer an electrically neutral atom -- they become positively charged zinc ions (Zn2+). Importantly, with the zinc, the electrons are not given to a different atom, they are sent out of the zinc, and into the circuit, powering it, and eventually returning to the electrolyte.
The Journey of Electrons
- When a zinc electrode is placed in a battery, the first electron leaves the zinc atom and enters the external circuit, starting its journey through the circuit. The external circuit can be a light bulb, a motor, anything that requires electricity.
- The electron then travels through the external circuit, providing the electric current that can be utilized to power devices or perform work.
- Eventually, the electron reaches the other electrode (cathode) of the battery, which is usually made of a different material such as copper.
- At the cathode, the electron combines with positive ions from the electrolyte, completing the circuit and maintaining charge balance.

Step 2: Preparation
For those of you who didn't fall asleep during the theory, let's start making our battery.
Attached to this step are two files. One is a base, and the other is a spring-loaded pressure plate, of which you'll need to print two. The pressure plates help everything stay compressed and in the center of the base. I could have designed this to only need one spring-loaded plate, but I wanted the cells to be in the middle of the base.
Go ahead and print the files and set them aside until after step 3.
Take your porous substance and cut it into pieces that are as big as your zinc/copper strips, which, for reference, are 50x20.2mm. I used folded-up tissues for my porous electrolyte. The tissues were four-ply and are folded up to be four layers, although it doesn't matter that much, as long as they're thick enough to separate the cells' layers.
If the tissues are too big, they could touch with the other layers and short out that cell.
If they are too small, the metals from one side of the cell could be touching the other, essentially also shorting the cell.
You want them to be just the right size and thickness.

Copper and Zinc strips. (Link)

Also, take two wires (~250mm each) and strip off 15-20mm off the ends. The sliding elements have holes for stripped wire to be threaded through and around, and if you have enough wire, you can loop it back around to keep it tied. Here's how the sliders should look:

Edit: By common request, I've added two more files, these are to make a voltaic pile without the need for these springs. This version is gravity-pressed, as the original voltaic pile was.
Last preparatory thing, take a 500mL bottle of water and pour 1/4 tsp of table salt in it. Label it, and set it aside.

I used some weird pink Himilayan salt because my wife is boujee and that's all she buys, but any kind of table salt will also work. It will be messier, but you could also use Gatorade, vinegar or lemon juice if you wanted.
Step 3: Voltaic Sandwiches
We create a battery cell by having one anode, one cathode, and our electrolyte. Each cell will have a fixed voltage defined by the characteristics of the components, but we can raise the voltage by creating several cells and connecting them in series, which is exactly how Volta's original pile was oriented.
Wiring circuits in series adds their individual voltages together. Series wiring can be accomplished by connecting the anode of one cell to the cathode of another, and using the remaining anode and cathode as if they were the only two terminals. Our voltaic pile will do this for us automatically, as long as we insert the layers and sandwiches in the right order.
What I call a "voltaic sandwich" is just one cell of the pile. From bottom-to-top, I put one zinc sheet, two tissue folds, and then a copper sheet. This makes one sandwich. I then put it into the voltaic pile such that the zinc is left and the copper is right. You can then add several more sandwiches, ensuring that you insert each one in the same orientation, with zinc on the left.
A 3-cell pile would be ordered this way, from left-to-right:
- Zinc
- Tissue
- Tissue
- Copper
- Zinc
- Tissue
- Tissue
- Copper
- Zinc
- Tissue
- Tissue
- Copper
Here's one voltaic sandwich:

Here's my test of one voltaic sandwich. It generated roughly 0.75 volts, and had a short circuit amperage of about 1-2mA, but take that amperage figure with a grain of salt (short circuit is not a standard use case).
Step 4: Full Stack
 The full stack
The full stack
For this step, I chose to change from facial tissues to bathroom tissues. I found that I could get them to the correct size with four folds and no cutting, and they were more absorbent. If I do this again, I might be inclined to use laser-cut cardboard sheets instead, but for the purposes of this project, pretty much anything will work. If you do want to laser cut something, I've included an SVG for some 50mm x 20.20mm rectangles that will fit the metal strips perfectly.
This project was my first time incorporating springs with 3D printed parts. I used these long, thin springs because they seemed to be able to compress far enough to allow the sandwiches to be inserted, but I didn't realize just how much force they require to be fully compressed. It's amazing the force that these little tiny springs can withstand.
I found it was easiest to put the leftmost pressure plate into position, insert all the voltaic sandwiches, and then pull everything back to insert the other pressure plate. Take a moment to ensure that your black/ground wire is touching zinc and your red/positive wire is touching copper. This is the correct configuration, and matching these to the red/black on your multimeter will ensure it displays values correctly, or, more importantly, it will ensure correct polarity in your circuit.
Once everything is finished, it will look like this:

But it's not generating any power yet!
I cut a small hole in the cap of my salt water bottle, which makes it easier to drip the electrolyte onto the sandwiches. If you're using the vertical version of the voltaic pile, you'll probably want to lie it down flat on its side until it's ready.
Also, I crimped the two terminals into a DuPont connector so that I could easily plug them into my breadboard, but this is obviously not required, just makes things easier.
Activating the Battery
From here, it's simple. Just get your circuit setup, and slowly drip your electrolyte (the salt water) onto your porous sheets. I did this very lazily -- if you spill, it might short circuit the battery for a few moments, but it's a low current and doesn't matter. In fact, it's kind of cool to see the LED flicker when the battery shorts out. Here's a video of me lighting an LED with all ten cells of my voltaic pile:
As you can see, I only got about 2.6v out of it. I have a feeling that some of my porous layers were extending beyond the dimensions of the metals, and were touching other cells, causing their voltages to not add as expected. I was expecting around 7 volts. The discrepancy could also be due to some diseconomy of scale that I am not aware of.
At any rate, it was enough to light the LED. It was very neat to see a completely closed system generating electricity in a way that I could see. Rechargable batteries make sense logically, you "fill" them up with electricity, and then they provide it later, but this was something different entirely -- this is a chemical reaction happening in real time. Technically most non-rechargable batteries are also chemical reactions, but there is something about one that is made by hand that makes it all the more enthralling.

Out of curiosity, I touched the two terminals to my tongue, and sure enough, I could feel the electricity as it bridged from one terminal to the other through my tongue. It was the exact same feeling as doing so with a common 9V battery, although of starkly lower magnitude.

Congratulations on making your voltaic pile! Alessandro Volta would be proud.
If you enjoyed this project, please consider supporting my future endeavors through Christopher's Factory:
Or by simply checking out my other content.
Thank you for reading, and please feel free to send me pictures of your voltaic pile or any questions you may have to christophersfactory@gmail.com
Thank you, Instructables' contest judging staff for your consideration.
Attachments

Runner Up in the
Battery-Powered Contest



















